- Weekly Highlights from Arakan State (Jan 26 to Feb 1, 2026)
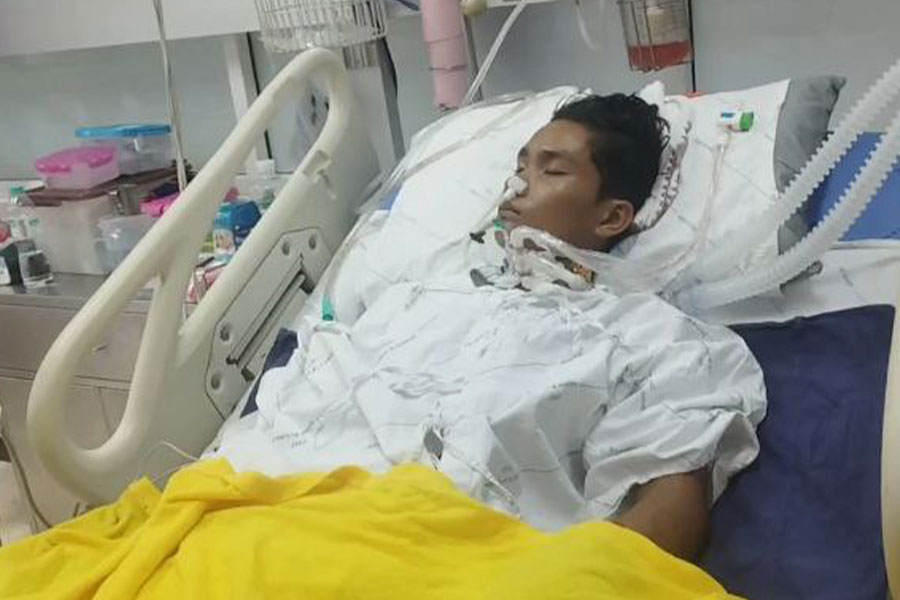
- Arakanese youth stabbed in Mae Sot urgently needs financial aid for medical treatment
- Five years on Myanmar faces uncertain military and political outlook after coup
- Myanmar Navy detains Pauktaw fishermen and demands ransom
- Junta Airstrikes on Arakan and the Consequences for Independent Media
Arakan State regulator to make follow-up checks for foods flagged as dangerous
The Arakan State Food and Drug Administration (FDA) will visit markets to control the sale of foods flagged by the FDA head office as unsuitable for human consumption.
17 Dec 2022
DMG Newsroom
17 December 2022, Sittwe
The Arakan State Food and Drug Administration (FDA) will visit markets to control the sale of foods flagged by the FDA head office as unsuitable for human consumption.
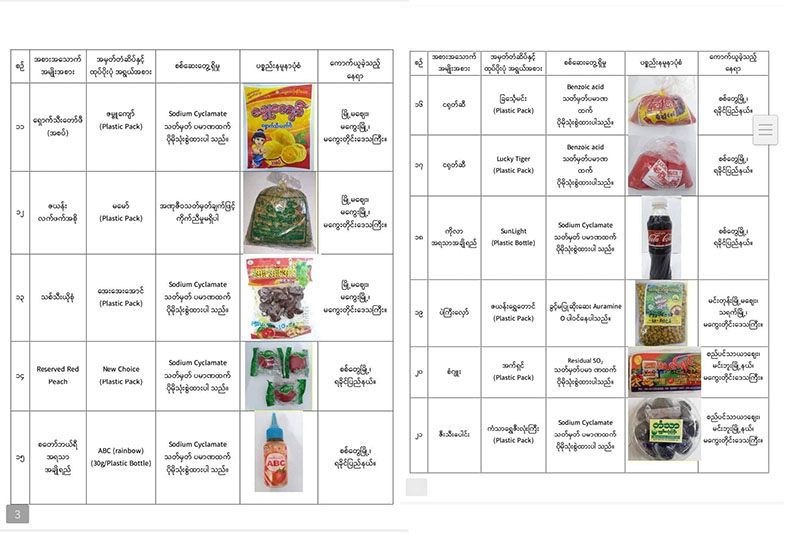
The FDA on August 30 announced a list of 39 brands of packed food that are dangerous for consumption because of the use of chemicals and prohibited artificial colours. Five of the brands are produced in the Arakan State capital Sittwe.
The Arakan State FDA will check if those brands are still sold in markets and take legal action against shop owners who continue to sell those brands, said staff officer Dr. Ye Min Thein of the Arakan State FDA.
“There can be health risks for people if they consume those products. So, we want the business owners to cooperate with us. We will make field visits soon, and take legal action against sellers,” he said.
Sellers will face prosecution under Section 28(a) of National Food Law, which carries a fine of K300,000 and/or up to three years in prison.
Among the foodstuffs produced in Sittwe and flagged by the FDA as unsuitable for consumption are two brands of soft drink, two brands of chili sauce and packed damson.
“The FDA needs to do more to make sure people know the names of those products, and producers should also halt their production,” said a resident of Rathedaung Township, Arakan State.
The Arakan State FDA must do more to educate consumers about those foods flagged as dangerous for consumption, agreed grocery store owner Ma Nyo Nyo from Sittwe.
The FDA branch in Arakan State regularly collects samples of foods that are popular with local consumers and lab tests them for safety.
Consumption of FDA-blacklisted foods can result in stomach, kidney and liver damage, and increases the likelihood of developing cancer over the long term, the regulator said.














.jpg)

